Describe How Understanding the Types of Chemical Reaction Is Useful
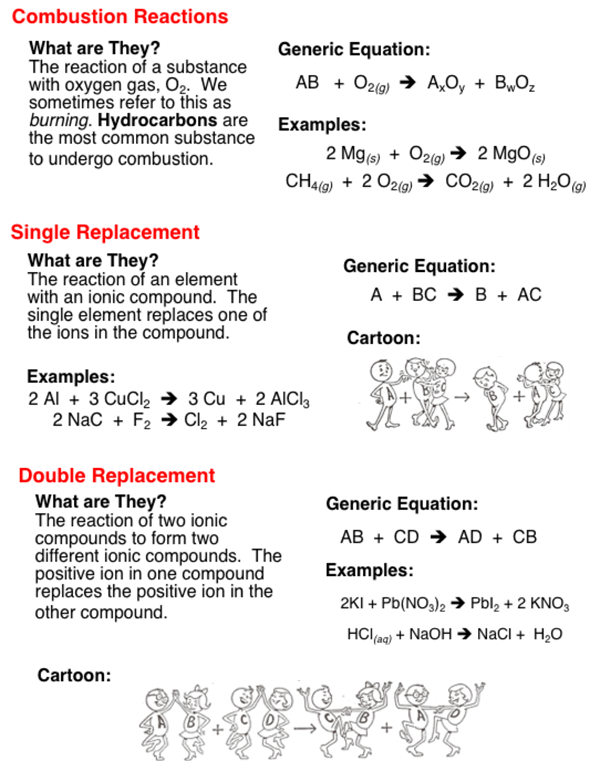
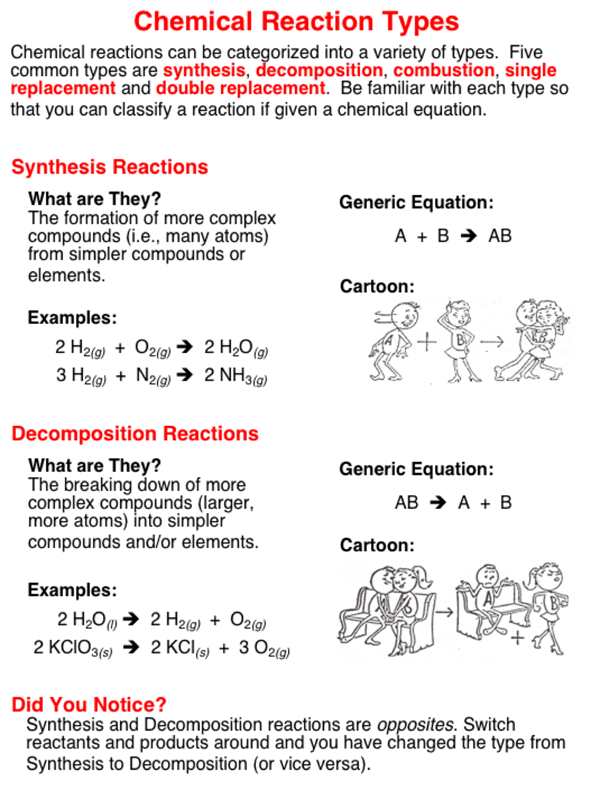
The five basic types of chemical reactions are combination decomposition single-replacement double-replacement and combustion. Chemical reactions can be classified into different categories.

What Are Chemical Equations Detailed Explanation Examples
Here they are in no particular order.

. This is the answer that could give a chemist without error to exaggerate. Start studying understanding chemical reactions. An example of a reaction that goes to completion is the reaction of hydrochloric acid with calcium carbonate in the form.
The different types of chemical reactions are vital for our culture technology and life. Using word or symbol equations to represent chemical reactions. Substances are either chemical elements or compounds.
Students will also practice balancing chemical equations. To denote stoichiometric relationships the symbol is used. The properties of the products are.
And is that someone anxious in this matter will try to see things from the molecular or atomic point of view will try to see reactions everywhere and molecules constantly transmuting. Use examples from the reactions studied Again by using examples from the reactions YOU studied to illustrate your argument explain Ihr words discuss the evidence poolumbyonoxiole 7. In other words the reaction continues until at least one of the reactants is completely consumed.
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. An arrow is drawn between the reactants and products to indicate the direction of the. Describe three kinds of chemical reactions.
Up to 24 cash back Purpose. Having a thorough understanding of these types of reactions will be useful for predicting the products of an unknown reaction. The chemical reactions We can find them in daily life in a general way.
Having a thorough understanding of these types of reactions will be useful for predicting the products of an unknown reaction. Copper II nitrate is blue and sodium hydroxide is clear. Multiple entities on either side of the reaction symbols describe above are separated from each other with the help of the symbol in a chemical equation.
This means that the total mass of the reactants is the same as the total mass of the products. Learn vocabulary terms and more with flashcards games and other study tools. Decomposition reactions are the opposite of combination reactions.
And exothermic chemical reactions are those chemical reactions that release heat and energy. A combustion reaction is when oxygen combines with another compound to form water and carbon dioxide. Endothermic chemical reactions are those chemical reactions that use heat and other energy.
Many chemical reactions can be classified as one of five basic types. Find step-by-step Anatomy and physiology solutions and your answer to the following textbook question. All chemical reactions can be placed into one of six categories.
The five basic types of chemical reactions are combination decomposition single-replacement double-replacement and combustion. 3H 2g N 2g 2NH 3g Hydrogen gas and nitrogen gas are combined in the presence of a catalyst at high temperature and pressure to produce ammonia gas. In an endothermic reaction more energy is required to break the bonds of the reactants than is released when the products form.
Different types of chemical reactions are used to produce a range of products and can occur at different rates investigating how chemistry can be used to produce a range of useful substances such as fuels metals and pharmaceuticals. In what a chemical reaction is how chemists represent reactions and describe the main features 5 types of reactions observed in this experiment. Many chemical reactions can be classified as one of five basic types.
In order to describe a reaction that occurs in both forward and backward directions the symbol is used. We balance complex chemical equations for getting desired results. When added together a precipitate will form and become more electric blue.
1 from And i realized that i have seen this scene before sometimes a little bowl of curry and rice or his favorite. Students will observe seven different chemical reactions identify the reactants and products of those reactions by both name and formula and classify the reactions as one of the five basic types of chemical reactions. Smelting iron burning fuels pottery and glass manufacturing production of cheese and wine and brewing processes are typical chemical reaction examples.
For each type of the reaction. Ten Important Chemical Reactions 1. A chemical formula contains __ symbols and __ to describe the makeup of a compound.
Chemical reactions result from the interactions of the electrons surrounding the atom. An example of this kind of reaction is the burning of napthalene. C 10 H 8 12 O 2.
People versed in chemistry can not avoid seeing things. The precipitate copper II hydroxide is heated until it turns black forming a new solid copper II oxide. Chemical reactions occur when chemical bonds between atoms are formed or broken.
A reaction in which two or more reactants combine to form a single product is known as a combination reaction. The law of _____ states that the total mass of the reactants before a chemical reaction is the same as the total mass of the. A chemical reaction is a process in which one or more substances also called reactants are converted to one or more different substances known as products.
The substances that go into a chemical reaction are called the reactants and the substances produced at the end of the reaction are known as the products. An exothermic reactionis a chemical reaction that releases thermal energy. These reactions are exothermic meaning they produce heat.
Different Types of Chemical Reactions. A thorough understanding of these types of reactions is useful for predicting the. Background For most of the reactions studied so far in chemistry you have probably assumed that the reaction goes to completion.
Synthesis of ammonia leads to the production of fertilizer ammonium nitrate and to the production of ammunitions. These interactions can include both releasing and breaking of chemical bonds and also. Chemical reactions that absorb thermal energy are endothermic reactions.
The 5 primary types of chemical reactions are. A neutralization reaction is a reaction colorredbetween an acid and a base Generally aqueous acid-base reactions produce water and a salt A combination reaction is a reaction in which colorredtwo or more substances combine to form a single product. This clarification is helpful for students to understand.

Types Of Chemical Reactions Chemistry Classroom Teaching Chemistry Science Lessons

Types Of Chemical Reactions Chemtalk

Types Of Chemical Reactions Detailed Explanation With Example Videos

What Is A Chemical Reaction Physical Vs Chemical Change Examples Chemtalk

What Is A Decomposition Reaction Definition And Examples

Chemical Reactions Definition Equations Types Examples With Faqs Of Chemical Reactions
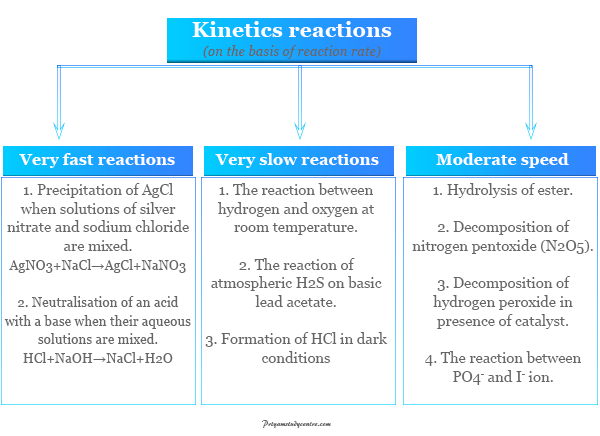
Chemical Kinetics Half Life Definition Formula Equations
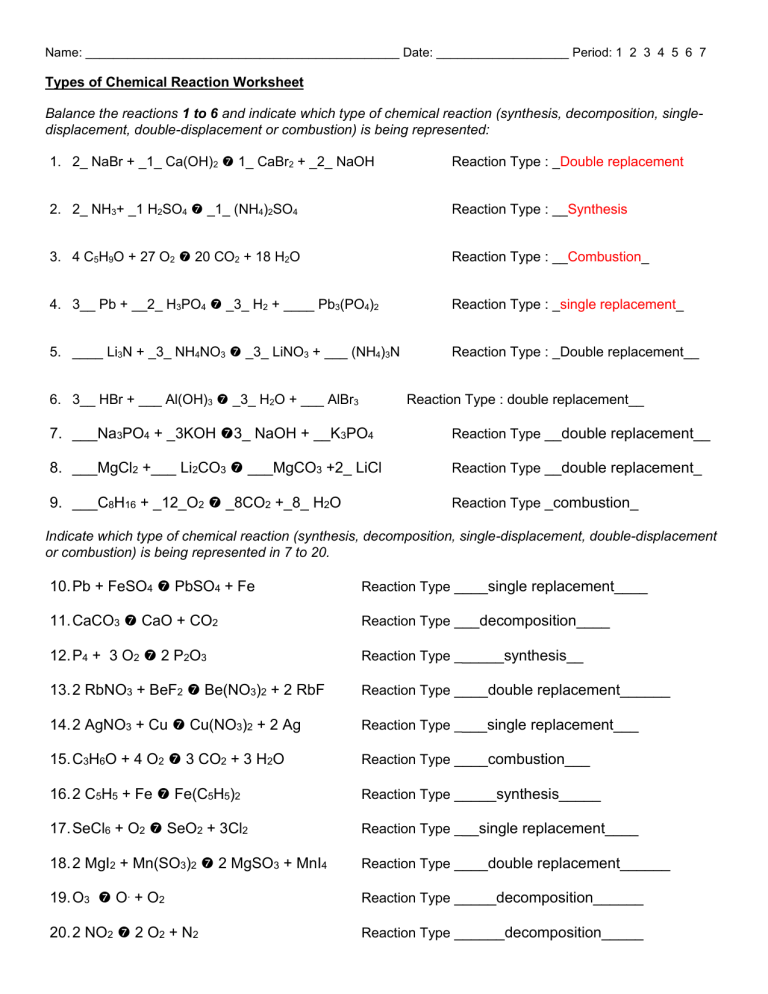
Types Of Chemical Reaction Worksheetanswers

Docstoc Make Your Business Better Chemistry Lessons Science Chemistry Teaching Chemistry
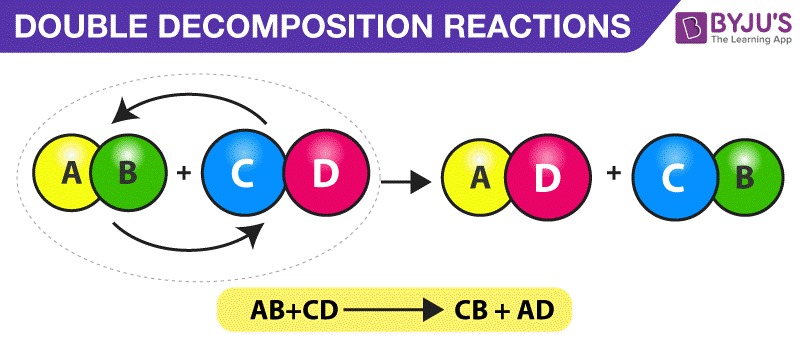
Decomposition Reaction Definition Types Examples Uses

Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
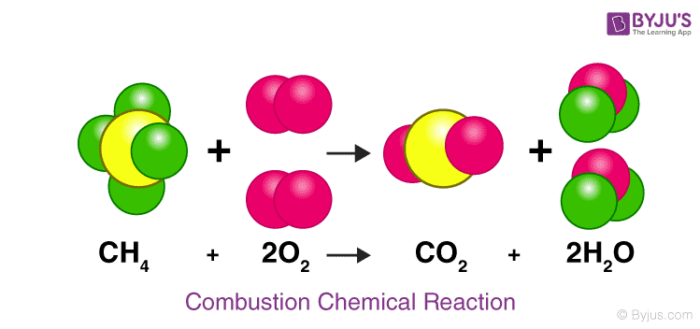
Chemical Reactions Definition Equations Types Examples With Faqs Of Chemical Reactions

What Is A Chemical Equation Definition And Examples

Chemical Reactions Introduction Video Khan Academy

Types Of Chemical Reactions With Examples Presentation And Handout Chemical Reactions Classroom Instruction Presentation


Comments
Post a Comment